UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| |
||||
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code: +
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading symbol |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
From time to time, Wave Life Sciences Ltd. (the “Company”) presents and/or distributes slides and presentations to the investment community to provide updates and summaries of its business. On November 12, 2020, the Company updated its corporate presentation, which is available on the “For Investors & Media” section of the Company’s website at http://ir.wavelifesciences.com/. This presentation is also furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Item 7.01 is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, nor shall it be deemed incorporated by reference into any registration statement or other filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
The following exhibit relating to Item 7.01 is furnished and not filed:
| Exhibit |
Description | |
| 99.1 | Corporate Presentation of Wave Life Sciences Ltd. dated November 12, 2020 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| WAVE LIFE SCIENCES LTD. | ||
| By: | /s/ Paul B. Bolno, M.D. | |
| Paul B. Bolno, M.D. | ||
| President and Chief Executive Officer | ||
Date: November 12, 2020

Wave Life Sciences Corporate Presentation November 12, 2020 Exhibit 99.1

Forward-looking statements This document contains forward-looking statements. All statements other than statements of historical facts contained in this document, including statements regarding possible or assumed future results of operations, preclinical and clinical studies, business strategies, research and development plans, collaborations and partnerships, regulatory activities and timing thereof, competitive position, potential growth opportunities, use of proceeds and the effects of competition are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause the actual results, performance or achievements of Wave Life Sciences Ltd. (the “Company”) to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this presentation are only predictions. The Company has based these forward-looking statements largely on its current expectations and projections about future events and financial trends that it believes may affect the Company’s business, financial condition and results of operations. These forward-looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, including those listed under Risk Factors in the Company’s Form 10-K and other filings with the SEC, some of which cannot be predicted or quantified and some of which are beyond the Company’s control. The events and circumstances reflected in the Company’s forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. Moreover, the Company operates in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that the Company may face. Except as required by applicable law, the Company does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.

Building a leading genetic medicines company ALS: Amyotrophic lateral sclerosis; FTD: Frontotemporal dementia 1stereopure oligonucleotides and novel backbone chemistry modifications Innovative platform Stereopure oligonucleotides Novel backbone modifications (PN chemistry) Allele-selectivity Multiple modalities (silencing, splicing, ADAR editing) Strong IP position1 Foundation of NEUROLOGY programs Huntington’s disease ALS / FTD Neuromuscular diseases Ataxias Parkinson’s disease Alzheimer’s disease Clinical development expertise Multiple global clinical trials ongoing across eight countries Innovative trial designs Manufacturing Established internal manufacturing capabilities to produce oligonucleotides at scale Wave’s drug discovery and development platform

PRISM has unlocked novel and proprietary advances in oligonucleotide design Backbone modifications Sugar modifications Drug approvals (FDA)2 1975 2020 2000 Mixtures of 2n molecules1 ~500,000 different molecules per dose fomivirsen pegaptanib Phosphorothioate (PS) mipomersen nusinersen PN backbone modification chemistry Stereopure backbone 2’-4’-cEt 2’-O-methyl 2’-F 2’-4’-LNA 1n=number of chiral centers 2’-MOE Phosphorodiamidate Morpholino (PMO) eteplirsen golodirsen givosiran patisiran inotersen viltolarsen 2oligonucleotide therapies approved by the FDA across the industry

THERAPEUTIC AREA / TARGET DISCOVERY PRECLINICAL CLINICAL PARTNER Huntington’s disease mHTT SNP1 Takeda 50:50 option Huntington’s disease mHTT SNP2 Huntington’s disease mHTT SNP3 ALS and FTD C9orf72 SCA3 ATXN3 CNS diseases Multiple† Takeda milestones & royalties DMD Exon 53 100% global ADAR editing Multiple AATD (ADAR editing) SERPINA1 100% global Retinal diseases USH2A and RhoP23H 100% global NEUROLOGY HEPATIC OPTHALMOLOGY WVE-003 WVE-004 WVE-120101 WVE-120102 Innovative pipeline led by neurology programs †During a four-year term, Wave and Takeda may collaborate on up to six preclinical targets at any one time. ALS: Amyotrophic lateral sclerosis; FTD: Frontotemporal dementia; SCA3: Spinocerebellar ataxia 3; CNS: Central nervous system; DMD: Duchenne muscular dystrophy; AATD: Alpha-1 antitrypsin deficiency Stereopure PN chemistry WVE-N531

WVE-120101 WVE-120102 WVE-003 Huntington’s Disease Portfolio

Huntington’s disease: a hereditary, fatal disorder Sources: Auerbach W, et al. Hum Mol Genet. 2001;10:2515-2523. Dragatsis I, et al. Nat Genet. 2000;26:300-306. Leavitt BR, et al. J Neurochem. 2006;96:1121-1129. Nasir J, et al. Cell. 1995;81:811-823. Reiner A, et al. J Neurosci. 2001;21:7608-7619. White JK, et al. Nat Genet. 1997;17:404-410. Zeitlin S, et al. Nat Genet. 1995;11:155-163. Carroll JB, et al. Mol Ther. 2011;19:2178-2185. HDSA ‘What is Huntington’s disease?’ https://hdsa.org/what-is-hd/overview-of-huntingtons-disease/ Accessed: 11/2/18.; Becanovic, K., et al., Nat Neurosci, 2015. 18(6): p. 807-16. Van Raamsdonk, J.M., et al., Hum Mol Genet, 2005. 14(10): p. 1379-92.; Van Raamsdonk, J.M., et al., BMC Neurosci, 2006. 7: p. 80. DNA CAG Repeat RNA wild-type (healthy) allele RNA mutant allele Normal CAG Repeat Expanded CAG Repeat Healthy protein (HTT) Mutant protein (mHTT) Neuro HD Autosomal dominant disease, characterized by cognitive decline, psychiatric illness and chorea; fatal No approved disease-modifying therapies Expanded CAG triplet repeat in HTT gene results in production of mutant huntingtin protein (mHTT); accumulation of mHTT causes progressive loss of neurons in the brain Wild-type (healthy) HTT protein critical for neuronal function; evidence suggests wild-type HTT loss of function plays a role in Huntington’s disease 30,000 people with Huntington’s disease in the US; another 200,000 at risk of developing the condition

Healthy CNS function Synaptic dysfunction | Cell death | Neurodegeneration mHTT toxic effects lead to neurodegeneration, loss of wtHTT functions may also contribute to HD Healthy individual Stresses wtHTT Huntington’s disease Stresses Toxic effects of mHTT + Loss of wtHTT functions ~50% decrease in wtHTT CNS, central nervous system; HD, Huntington’s disease; HTT, huntingtin protein; mHTT, mutant huntingtin protein; wtHTT, wild-type huntingtin protein. 1. Ross CA, Tabrizi SJ. Lancet Neurol. 2011;10(1):83-98. 2. Saudou F, Humbert S. Neuron. 2016;89(5):910-926. 3. Cattaneo E, et al. Nat Rev Neurosci. 2005;6(12):919-930. 4. Milnerwood AJ, Raymond LA. Trends Neurosci. 2010;33(11):513-523. Neuro HD

Plays an essential role in the transport of synaptic proteins—including neurotransmitters and receptors—to their correct location at synapses9-12 Promotes neuronal survival by protecting against stress (e.g., excitotoxicity, oxidative stress, toxic mHTT aggregates)1-8 BRAIN CIRCUITS SYNAPSE NEURON CSF circulation Supplies BDNF to the striatum to ensure neuronal survival13-16 Regulates synaptic plasticity, which underlies learning and memory17-22 Plays a critical role in formation and function of cilia—sensory organelles that control the flow of CSF—which are needed to clear catabolites and maintain homeostasis23 HD: Wild-type HTT is a critical protein for important functions in the central nervous system BDNF, brain-derived neurotrophic factor; CSF, cerebrospinal fluid; mHTT, mutant huntingtin protein. Sources: 1. Leavitt 2006 2. Cattaneo 2005 3. Kumar 2016 4. Franco-Iborra 2020 5. Hamilton 2015 6. Ochaba 2014 7. Wong 2014 8. Rui 2015 9. Caviston 2007 10. Twelvetrees 2010 11. Strehlow 2007 12. Milnerwood 2010 13. Smith-Dijak 2019 14. Tousley 2019 15. Zhang 2018 16. McAdam 2020 17. Altar 1997 18. Zuccato 2001 19. Gauthier 2004 20. Ferrer 2000 21. Baquet 2004 22. Liu 2011 23. Karam 2015 Neuro HD

Nature publication contributes to weight of evidence on importance of wild-type huntingtin Source: Poplawski et al., Nature, April 2019 Htt: Huntingtin protein Conditional knock-out of Htt in 4-month old mice (post-neuronal development) Results suggest that: Htt plays a central role in the regenerating transcriptome (potentially influencing genes such as NFKB, STAT3, BDNF) Htt is essential for regeneration Indeed, conditional gene deletion showed that Htt is required for neuronal repair. Throughout life, neuronal maintenance and repair are essential to support adequate cellular functioning Neuro HD

Utilize association between single nucleotide polymorphisms (SNPs) and genetic mutations to specifically target errors in genetic disorders, including Huntington’s disease (HD) Potential to provide treatment for up to 80% of HD population Wave approach: novel, allele-selective silencing Source: Kay, et al. Personalized gene silencing therapeutics for Huntington disease. Clin Genet. 2014;86:29–36. Neuro HD Aims to lower mHTT transcript while leaving healthy wild-type HTT relatively intact Allele-selectivity possible by targeting SNPs associated with expanded long CAG repeat in HTT gene RNase H and ASO:RNA RNA mutant allele

WVE-120101: Selective reduction of mHTT mRNA and protein Reporter Cell Line* Neuro HD Source: Meena, Zboray L, Svrzikapa N, et al. Selectivity and biodistribution of WVE-120101, a potential antisense oligonucleotide therapy for the treatment of Huntington’s disease. Paper presented at: 69th Annual Meeting of the American Academy of Neurology; April 28, 2017; Boston, MA.

Demonstrated delivery to brain tissue WVE-120101 and WVE-120102 distribution in cynomolgus non-human primate brain following intrathecal bolus injection In Situ Hybridization ViewRNA stained tissue Red dots are WVE-120102 oligonucleotide Arrow points to nuclear and perinuclear distribution of WVE-120102 in caudate nucleus Red dots are WVE-120101 oligonucleotide Arrow points to nuclear and perinuclear distribution of WVE- 120101 in cingulate cortex CIC = cingulate cortex In Situ Hybridization ViewRNA stained tissue Neuro HD CN = caudate nucleus Source: Meena, Zboray L, Svrzikapa N, et al. Selectivity and biodistribution of WVE-120101, a potential antisense oligonucleotide therapy for the treatment of Huntington’s disease. Paper presented at: 69th Annual Meeting of the American Academy of Neurology; April 28, 2017; Boston, MA.

PRECISION-HD clinical trials Single Dose Multidose 196 1 Washout CSF sample Dose 28 56 84 112 Study Day* 140 OLE 2 mg 4 mg 8 mg 16 mg 32 mg Multidose Cohorts (N = 12 per cohort) Trial results expected in 1Q 2021 OLE: Open label extension; CSF: cerebrospinal fluid; mHTT: mutant huntingtin; wtHTT: wild-type HTT; tHTT: total HTT * Study day may vary depending on patient washout period 1Hodges-Lehmann non-parametric shift estimates of the difference between treatment and placebo, p<0.05 (Wilcoxon-Mann-Whitney non-parametric significance test); 2Multiple Contrast Test (MCT), p=0.03; Interim data announced December 2019 Results Two Phase 1b/2a clinical trials for WVE-120101 and WVE-120102 ongoing Patients are migrated to highest dose tested Neuro HD Safety and tolerability Biomarkers mHTT Assay development work to measure wtHTT in CSF ongoing tHTT PRECISION-HD1 and OLE PRECISION-HD2 and OLE NfL

WVE-003 (SNP3) demonstrates selective, potent, and durable reduction of mHTT in preclinical models Selectively reduces mHTT mRNA in HD iPSC neurons in vitro Results from ND50036 iPSC-derived medium spiny neurons. Total HTT knockdown quantified by qPCR and normalized to HPRT1 Oligonucleotide or PBS [100 μg ICV injections through a cannula on days 1, 3, and 5] delivered to BACHD transgenic. Mean ± SD (n=8, *P<0.0332, ***P<0.0002, ****P<0.0001 versus PBS unless otherwise noted). HPRT1, hypoxanthine-guanine phosphoribosyl transferase; iPSC, induced pluripotent stem cell; ICV, intracerebroventricular; PBS, phosphate-buffered saline Similar results in cortex Pan-silencing reference compound WVE-003 PBS Weeks *** **** **** **** **** **** CTA submission expected in 4Q 2020 Pan-silencing reference compound WVE-003 Percentage HTT mRNA Remaining Durable striatal mHTT knockdown for 12 weeks in BACHD mouse model Neuro HD Incorporates PN backbone chemistry

Three allele-selective HD programs Intend to explore efficacy in early manifest and pre-manifest HD patient populations Neuro HD Potential to address ~80% of HD patient population % Huntington’s Disease Patient Population with SNP SNP1 WVE-120101 SNP2 WVE-120102 SNP3 WVE-003 SNP1 SNP2 SNP1 SNP2 SNP3 ~50% ~50% ~40% ~70%1 ~80%2 +10% of HD patients vs. SNP1 + SNP2 1 Percentage of patient population with SNP1 and/or SNP2 2 Percentage of patient population with SNP1, SNP2 and/or SNP3

WVE-004 Amyotrophic Lateral Sclerosis (ALS) Frontotemporal Dementia (FTD)

C9orf72 repeat expansions: A critical genetic driver of ALS and FTD Normal (non-expanded) Allele < 25 GGGGCC repeats Expanded Allele Sources: DeJesus-Hernandez et al, Neuron, 2011. Renton et al, Neuron, 2011. Zhu et al, Nature Neuroscience, May 2020 Typically 100’s-1000’s of GGGGCC repeats C9orf72 hexanucleotide repeat expansions (GGGGCC) are the strongest known risk factor for sporadic and inherited forms of Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Dementia (FTD) The C9orf72 repeat expansions also lead to accumulation of repeat-containing transcripts, nuclear sequestration of RNA binding proteins and synthesis of toxic dipeptide-repeat (DPR) proteins The C9orf72 repeat expansions lead to reduced expression of wild-type C9orf72 and to cellular changes that reduce neuronal viability Neuro C9orf72

C9-ALS and C9-FTD: Manifestations of a clinical spectrum Disease C9 specific US population Mean disease duration Standard of care C9-ALS Fatal neurodegenerative disease Progressive degeneration of motor neurons in brain and spinal cord ~2,000 3.1 years Significant unmet need despite two approved therapies in US C9-FTD Progressive neuronal atrophy in frontal/temporal cortices Personality and behavioral changes, gradual impairment of language skills ~10,000 6.4 years No approved disease modifying therapies Two devastating diseases with a shared genetic basis ALS: Amyotrophic lateral sclerosis; FTD: Frontotemporal dementia Sources: Cammack et al, Neurology, October 2019. Moore et al, Lancet Neurology, February 2020 Neuro C9orf72

C9orf72 repeat expansions: Mechanisms of cellular toxicity C9-ALS and C9-FTD may be caused by multiple factors: Insufficient levels of C9orf72 protein Accumulation of repeat-containing RNA transcripts Accumulation of aberrantly translated DPR proteins Recent evidence suggests lowering C9orf72 protein exacerbates DPR-dependent toxicity Sources: Gitler et al, Brain Research, September 2016. Zhu et al, Nature Neuroscience, May 2020 Targeted by Wave ASOs Variant-selective targeting could address multiple potential drivers of toxicity Neuro C9orf72
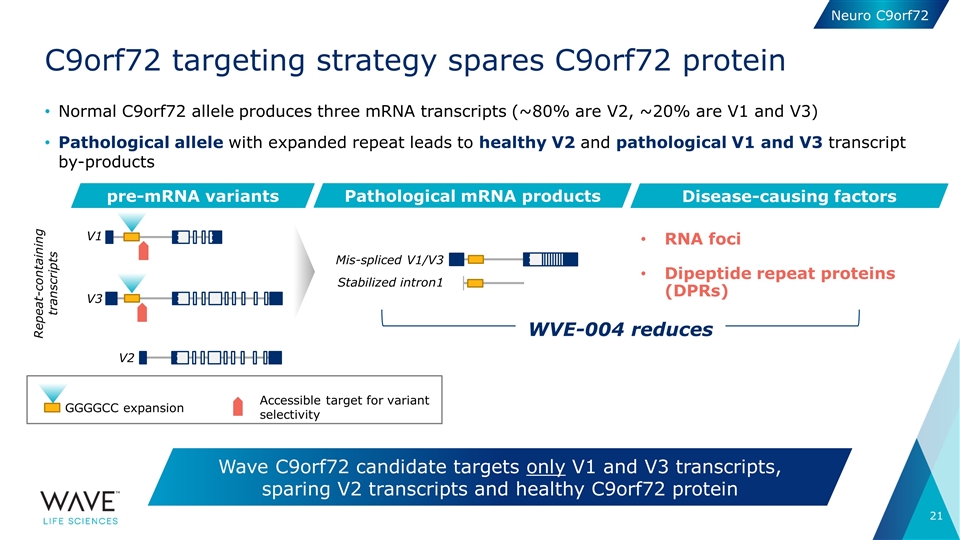
Normal C9orf72 allele produces three mRNA transcripts (~80% are V2, ~20% are V1 and V3) Pathological allele with expanded repeat leads to healthy V2 and pathological V1 and V3 transcript by-products C9orf72 targeting strategy spares C9orf72 protein Wave C9orf72 candidate targets only V1 and V3 transcripts, sparing V2 transcripts and healthy C9orf72 protein pre-mRNA variants Pathological mRNA products V1 V2 Mis-spliced V1/V3 Stabilized intron1 V3 Disease-causing factors RNA foci Dipeptide repeat proteins (DPRs) GGGGCC expansion Accessible target for variant selectivity WVE-004 reduces Repeat-containing transcripts Neuro C9orf72

PN backbone chemistry: Improved potency among C9orf72-targeting oligonucleotides in vivo Exposure (µg/g) Exposure (µg/g) C9orf72 compounds Spinal cord Cortex PS/PO backbone PS/PO/PN backbone %C9orf72 V3 transcript remaining Mice received 2 x 50 ug ICV doses on days 0 & 7; mRNA from spinal cord and cortex quantified by PCR (Taqman assay) 8 weeks later. Oligonucleotide concentrations quantified by hybridization ELISA. Graphs show robust best fit lines with 95% confidence intervals (shading) for PK-PD analysis. Spinal Cord Neuro C9orf72

WVE-004: Potent and selective knockdown of repeat-containing transcripts in vitro V3 Dose (μM) All V WVE-004 NTC Dose (μM) In vitro activity in C9 patient-derived neurons WVE-004 NTC Dose (μM) IC50:201.7nM In vitro selectivity in C9 patient-derived neurons C9 patient-derived motor neurons were treated with C9orf72 candidate and NTC under gymnotic conditions up to 10uM. Taqman qPCR assays were used to evaluating V3 and all V transcripts. NTC- non-targeting control. Relative fold change C9orf72 V3/HPRT1 1.5 1.0 0.5 0.0 0.001 0.01 0.1 1 10 Relative fold change C9orf72 V3/HPRT1 0.016 0.08 0.4 2 10 0.016 0.08 0.4 2 10 0.016 0.08 0.4 2 10 0.016 0.08 0.4 2 10 1.5 1.0 0.5 0.0 1.5 1.0 0.5 0.0 Relative fold change C9orf72/HPRT1 Neuro C9orf72

4 12 18 12 18 24 4 24 Durable knockdown of repeat transcripts in vivo after 6 months in spinal cord and cortex Spinal cord Cortex 1.0 0.5 0.0 Relative fold change C9orf72 V3/mHPRT1 WVE-004 PBS week 4 12 18 12 18 24 4 24 1.0 0.5 0.0 Relative fold change C9orf72 V3/mHPRT1 WVE-004 PBS week Experimental description: 2 x 50 ug on day 0 and day 7 dosed ICV; mRNA Samples were analyzed using quantitative PCR (Taqman assay) p≤0.0001 p≤0.0001 Neuro C9orf72

WVE-004 demonstrates durable reduction of DPRs in vivo after 6 months in spinal cord and cortex 4 12 18 12 18 24 4 24 Spinal cord Cortex 1.5 0.5 0.0 WVE-004 PBS week 4 12 18 12 18 24 4 24 WVE-004 PBS week 1.0 Relative Poly-GP levels (normalized to PBS) 1.5 0.5 0.0 1.0 Experimental description: 2 x 50 ug on day 0 and day 7 dosed ICV; DPRs were measured by Poly-GP MSD assay. *: p≤ 0.05 **: P ≤ 0.01, ***: P ≤ 0.001 ICV: intracerebroventricular; Dipeptide repeat proteins: DPRs p≤0.0001 * *** ** Relative Poly-GP levels (normalized to PBS) Neuro C9orf72

Healthy C9 protein relatively unchanged ~6 months after WVE-004 administration ns ns C9 BAC transgenic mice were administered PBS or 50 ug WVE-004, ICV, on day 0 and again on day 7. Relative fold change of total human C9orf72 to mouse Hprt1 protein in the spinal cord (left) and cortex (right) shown at 24 weeks after first administration. Data show mean ± SD (n=7). ns, not significant; PBS, phosphate-buffered saline; ICV: intracerebroventricular Spinal cord Cortex Relative fold change C9orf72/HPRT1 Relative fold change C9orf72/HPRT1 WVE-004 PBS WVE-004 PBS Neuro C9orf72

WVE-004 proof-of-concept study to include both ALS and FTD patients Patients with documented C9orf72 expansion and confirmed ALS or FTD diagnosis Single and multiple ascending doses to be explored Safety and tolerability Pharmacodynamic effects on key biomarkers while on treatment PolyGP NfL Key exploratory clinical outcome measures ALSFRS-R and CDR-FTLD CTA submission expected in 4Q 2020 CTA: clinical trial application; NfL: neurofilament light chain; ALSFRS-R: Amyotrophic Lateral Sclerosis Functional Rating Scale; CDRFTLD: Clinical Dementia Scale – frontotemporal lobar degeneration Neuro C9orf72

WVE-N531 Duchenne muscular dystrophy

WVE-N531 in vitro dose-dependent dystrophin restoration WVE-N531 contains novel PN chemistry modification Free uptake for 6 days in differentiation media with no transfection agent and no peptide conjugated to the oligonucleotide Demonstrated a dose-dependent increase in dystrophin restoration in DMD patient-derived myoblasts Experimental conditions: Δ45-52 (D45-52) patient myoblasts were treated with oligonucleotide for 6d under free-uptake conditions in differentiation media. Protein harvested in RIPA buffer and dystrophin restoration analyzed by Western Blot. Signal normalized to vinculin loading control and to primary healthy human myotube lysate (pooled from four donors) forming a standard curve in Δ45-52 cell lysate. Western Blot normalized to primary healthy human myoblast lysate Dystrophin protein restoration of up to 71% Neuro DMD

Substantial increase in survival observed in DKO model using PN chemistry (study ongoing) Double knock-out (DKO) mice lack dystrophin and utrophin protein and have a severe phenotype. Mdx/utr-/- mice received weekly subcutaneous (SC) 150 mg/kg dose of PS/PO or bi-weekly SC 75 mg/kg PS/PO/PN stereopure oligonucleotide beginning at postnatal day 10. Age-matched mdx/utr-/- littermates were treated with PBS, and mdx mice were not treated. Mice with severe disease were euthanized. DKO: PS/PO/PN 75 mg/kg n=9; PS/PO n=9, PBS n=12 PBS PS/PO, QW 150 mg/kg weekly PS/PO/PN, Q2W 75 mg/kg bi-weekly DKO Survival Neuro DMD

Planning underway for clinical trial investigating WVE-N531 in DMD DKO data and previously generated preclinical data support advancing WVE-N531 to the clinic Unmet need in DMD remains high Support from DMD advocacy community to explore possibility to improve efficiency of exon skipping with novel therapeutic approaches such as PN chemistry Planned clinical trial adequately powered to evaluate change in dystrophin production, drug concentration in muscle, and initial safety Open-label study; targeting every-other-week administration in up to 15 boys with DMD Trial planned to be conducted in Europe Potential to apply PN chemistry to other exons if successful CTA submission expected in 1Q 2021 Neuro DMD

Wave’s discovery and drug development platform

Through iterative analysis of in vitro and in vivo outcomes and machine learning-driven predictive modeling, Wave continues to define design principles that are deployed across programs to rapidly develop and manufacture clinical candidates that meet pre-defined product profiles Multiple modalities Silencing | Splicing | ADAR editing DESIGN Unique ability to construct stereopure oligonucleotides with one defined and consistent profile Enables Wave to target genetically defined diseases with stereopure oligonucleotides across multiple therapeutic modalities OPTIMIZE A deep understanding of how the interplay among oligonucleotide sequence, chemistry, and backbone stereochemistry impacts key pharmacological properties SEQUENCE STEREOCHEMISTRY CHEMISTRY Sequence Stereochemistry Chemistry

Sequence Stereochemistry Chemistry PRISM platform enables rational drug design Chemistry R: 2’ modifications OMe, MOE, F, other modifications 5’ 2’ 3’ 5’ 3’ 2’ R X B B X: backbone chemistry Phosphodiester (PO), phosphorothioate (PS), Phosphoramidate diester (PN) Sequence B: bases A, T, C, mC, G, U, other modified bases Stereochemistry Chiral control of any stereocenter 5’ modifications, backbone modifications

Backbone modification (X) Phosphodiester Phosphorothioate Phosphoramidate diester Stereochemistry Not chiral Chiral Chiral Charge Negative Negative Neutral Depiction PRISM backbone modifications Expanding repertoire of backbone modifications with novel PN backbone chemistry Molecule structure illustrative of backbone modification patterns Backbone linkages PS PO PN PO/PS PO/PS/PN Phosphoryl guanidine x-ray structure Stereorandom PS backbone Rp PS backbone Sp PN backbone Sp PN backbone Rp PN backbone Stereorandom

Silencing Splicing In vitro knockdown of PS/PO containing compounds compared to PS/PN compounds with same sequence and PS stereochemistry Rational design using PN chemistry backbone modification increases in vitro potency in most cases Presented at Analyst & Investor Research Webcast on August 25, 2020; Left: Experiment was performed in iPSC-derived neurons in vitro; target mRNA levels were monitored using qPCR against a control gene (HPRT1) using a linear model equivalent of the DDCt method; Right: DMD patient-derived myoblasts treated with PS/PO or PS/PO/PN stereopure oligonucleotide under free-uptake conditions. Exon-skipping efficiency evaluated by qPCR. PS/PO compounds are rank-ordered on X-axis. PS/PO reference compound PS/PN modified compound In vitro skipping efficiency of PS/PO containing compounds compared to PS/PO/PN compounds with same sequence and PS stereochemistry PS/PO reference compound PS/PO/PN modified compound Ranked by potency of reference PS/PO compound Ranked by potency of reference PS/PO compound Target knockdown (% remaining) Improved knockdown Improved skipping % skipping

Lead program in Takeda collaboration reinforces potential of PN chemistry in the CNS Single IT dose of 12 mg (n=3) Therapeutic candidate widely distributed across brain and spinal cord ~90% mRNA knockdown one-month following single dose Substantial and widespread target mRNA reduction following single intrathecal dose in NHPs NHPs: Non-human primates; IT: intrathecal NHPs were administered 12 mg on day 1 via IT bolus injection; tissue samples were collected from 3 NHPs at 28 days post-dose. Target mRNA knockdown 28 days post-dose

PRISM enables optimal placement of backbone stereochemistry Crystal structure confirms phosphate-binding pocket of RNase H binds 3’-SSR-5’ motif in stereopure oligonucleotide – supports design strategy for Wave oligonucleotides ASO/RNA duplex Yellow spheres represent ‘S’ atoms Phosphate binding pocket RNA cleavage site Target RNA Stereopure Oligonucleotide (C9orf72 compound) RNase H + +

Importance of controlling stereochemistry (Rp) (Sp) Top view Side view Yellow spheres represent ‘S’ atomsPS: Phosphorothioate Number of PS linkages in oligonucleotide backbone No. diastereomers 80T 60T 40T 20T 30B 22M 12M 2M 1M 500K 0 0 10 20 30 40 50 Antisense, exon skipping, ssRNAi ADAR oligonucleotide CRISPR guide Stereochemical diversity Exponential diversity arises from uncontrolled stereochemistry

ADAR editing Platform capability and Alpha-1 antitrypsin deficiency

PRISM platform has unlocked ADAR editing A-to-I editing is one of most common post-transcriptional modifications ADAR is ubiquitously expressed across tissues, including liver and CNS ADAR Target RNA I(G) A Edited RNA Oligonucleotide establishes double-stranded RNA complex Oligonucleotide Modification Delivery A: adenosine; I: inosine; G: guanosine; Nishikura, K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol. 2016; Picardi, E. et al. Profiling RNA editing in human tissues: towards the inosinome Atlas. Scientific reports 5, 14941, doi:10.1038/srep14941 (2015). ADAR editing

PRISM enables practical approach to RNA editing without need for viruses or exogenous protein Intracellular Extracellular space Endogenous ADAR Unedited RNA Wave ADAR-editing Oligonucleotides Exogenous ADAR or other base editors Edited RNA Protein release/ expression Delivery vehicles Alternative Base-Editing Systems Edited RNA Genetic construct or foreign protein ADAR editing

Wave platform Fully chemically modified to increase stability in vivo Chirally-controlled backbone to maximize in vitro activity PN backbone chemistry modification improves editing efficiency No requirement for AAV / nanoparticles GalNAc-conjugated for targeted delivery into liver Avoids permanent off-target DNA edits No immunogenicity from exogenous proteins Reduced off-target effects Advantages of Wave ADAR editing platform Sources: Chen Biochemistry 2019 Chemically modified Simplified delivery Endogenous ADAR ADAR editing
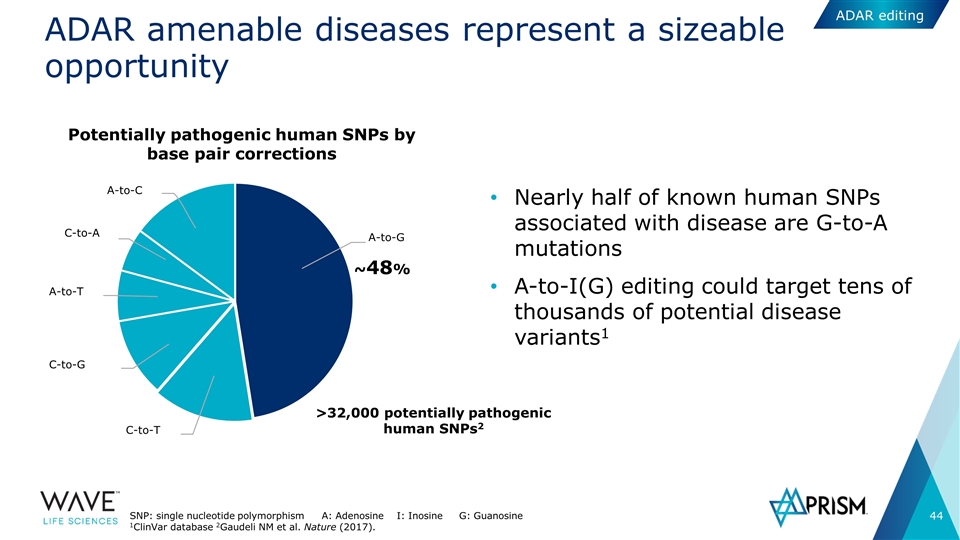
ADAR amenable diseases represent a sizeable opportunity Nearly half of known human SNPs associated with disease are G-to-A mutations A-to-I(G) editing could target tens of thousands of potential disease variants1 ~48% C-to-T C-to-G A-to-T C-to-A A-to-C A-to-G Potentially pathogenic human SNPs by base pair corrections >32,000 potentially pathogenic human SNPs2 SNP: single nucleotide polymorphism A: Adenosine I: Inosine G: Guanosine 1ClinVar database 2Gaudeli NM et al. Nature (2017). ADAR editing

RNA editing opens many new therapeutic applications Fix nonsense and missense mutations that cannot be splice-corrected Remove stop mutations Prevent protein misfolding and aggregation Alter protein processing (e.g. protease cleavage sites) Protein-protein interactions domains Modulate signaling pathways miRNA target site modification Modifying upstream ORFs Modification of ubiquitination sites Restore protein function Recessive or dominant genetically defined diseases Modify protein function Ion channel permeability Protein upregulation Haploinsufficient diseases Examples: Examples: Examples: ADAR editing

Data from independent experiments; Total RNA was harvested, reverse transcribed to generate cDNA, and the editing target site was amplified by PCR and quantified by Sanger sequencing PN chemistry improves editing efficiency PN backbone modification increased both potency and editing efficiency in vitro ACTB editing in primary human hepatocytes using GalNAc-mediated uptake ADAR editing

Significant ADAR editing demonstrated in vitro in NHP and primary human hepatocytes NHP: non-human primate; ACTB: Beta-actin; nd= not determined Total RNA was harvested, reverse transcribed to generate cDNA, and the editing target site was amplified by PCR. In vitro dose-response human hepatocytes In vitro dose-response NHP hepatocytes % Editing % Editing ACTB 1 ACTB 2 ACTB 3 ACTB 1 ACTB 2 ACTB 3 ACTB GalNAc-conjugated oligonucleotides with stereopure PN chemistry modification ADAR editing

Efficient ADAR editing translated in vivo in non-human primate study NHP: non-human primate; ACTB: Beta-actin; Left: 5mg/kg SC: Day 1,2,3,4,5; Liver Biopsy for mRNA (ACTB Editing) & eASO Exposure: Day 7 Up to 50% editing efficiency observed at Day 7, 2 days post last dose Substantial and durable editing out to at least Day 50, 45 days post last dose In vivo editing in NHP following subcutaneous administration Oligonucleotide quantification in NHP following subcutaneous administration 2 days post last dose 45 days post last dose 2 days post last dose 45 days post last dose ACTB 1 ACTB 2 ACTB 3 ACTB 1 ACTB 2 ACTB 3 Untreated (pre dose) % Editing µg of oligonucleotide per gram of tissue ADAR editing

Editing site RNA editing within ACTB transcript (human hepatocytes) RNA editing within transcriptome (human hepatocytes) Wave ADAR editing oligonucleotides are highly specific Coverage Genome coordinates ACTB C 0% T 100% C 53.8% T 46.2% ACTB Confidence (LOD score) % Editing Mock Editing oligonucleotide Human hepatocytes were dosed with 1um oligonucleotide, 48 hours later RNA was collected and sent for RNA sequencing. RNAseq conducted using strand-specific libraries to quantify on-target ACTB editing and off-target editing in primary human hepatocytes; plotted circles represent sites with LOD>3 ADAR editing
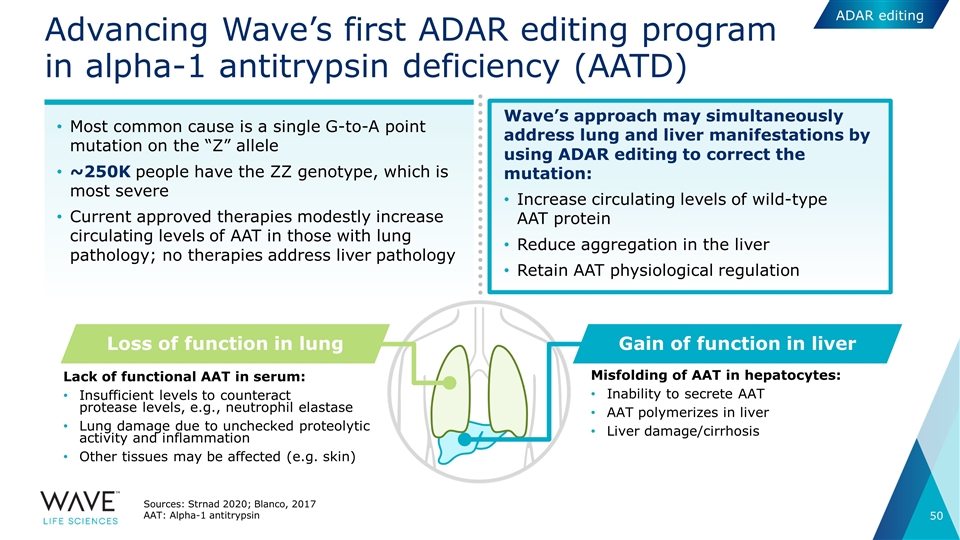
ADAR editing Advancing Wave’s first ADAR editing program in alpha-1 antitrypsin deficiency (AATD) Sources: Strnad 2020; Blanco, 2017 AAT: Alpha-1 antitrypsin Most common cause is a single G-to-A point mutation on the “Z” allele ~250K people have the ZZ genotype, which is most severe Current approved therapies modestly increase circulating levels of AAT in those with lung pathology; no therapies address liver pathology Loss of function in lung Gain of function in liver Lack of functional AAT in serum: Insufficient levels to counteract protease levels, e.g., neutrophil elastase Lung damage due to unchecked proteolytic activity and inflammation Other tissues may be affected (e.g. skin) Misfolding of AAT in hepatocytes: Inability to secrete AAT AAT polymerizes in liver Liver damage/cirrhosis Wave’s approach may simultaneously address lung and liver manifestations by using ADAR editing to correct the mutation: Increase circulating levels of wild-type AAT protein Reduce aggregation in the liver Retain AAT physiological regulation

SERPINA1 RNA editing increases protein concentration in vitro Mouse primary hepatocytes that express SERPINA1-PIZ allele were transfected with 25 nanomolar (nM) of SERPINA1 (SA1-1 and SA1-2) targeting antisense oligonucleotides (ASOs) and a control non-targeting (NT) ASO. Media and RNA was collected at 5 days post transfection. SerpinA1 Protein in media was quantified by Elisa Assay, RNA editing was quantified by RT/PCR/Sanger sequencing. All samples done at N=6 replicates. In primary hepatocyte Pi*Z cell model, editing the Z transcript back to wild-type prevents protein misfolding and increases secretion from hepatocytes 3-Fold Increase SerpinA1 Protein Concentration in Media SERPINA1 mRNA Editing % SERPINA1-Pi*Z mRNA Editing SerpinA1 Protein ng/ml ADAR editing

Proprietary humanized mouse model developed to support ADAR platform Model validation and in vivo data expected 1H 2021 SERPINA1-Pi*Z/huADAR Protein ü Human ADAR Expressed in all tissues huADAR mouse Protein Pathology ü huADAR Liver pathology, lower huSERPINA1 serum ü SERPINA1 SERPINA1 mouse Protein ü huSERPINA1-Pi*Z Expressed in liver Expression of huADAR in mouse is comparable to expression in human cells Expression of huADAR restores editing of endogenous targets in primary mouse cell types to levels seen in human primary cell types huADAR mouse model can be crossed with disease specific mouse models to provide model systems for use across Wave’s ADAR editing programs ADAR editing

Multiple opportunities for ADAR editing in neurology ACTB editing in iCell Neurons ACTB editing in human iCell Astrocytes Concentration (µM) % Editing % Editing Compound 2 (PS / PN) Compound 1 (PS / PN) Compound 3 (PS / PN) EC50: ~200-250nM Gymnotic uptake; Total RNA was harvested, reverse transcribed to generate cDNA, and the editing target site was amplified by PCR and quantified by Sanger sequencing Concentration (µM) ADAR editing hADAR: human ADAR; UGP2: Glucose Pyrophosphorylase 2; 5 mice in each group were injected with PBS or a single 100uG dose on day 0. Animals were necropsied on day 7. RNA was harvested and editing measured by Sanger sequencing. In vivo CNS editing in proprietary hADAR transgenic mouse (1 week)

Ophthalmology

Stereopure oligonucleotides for inherited retinal diseases (IRDs) Wave ophthalmology opportunity Oligonucleotides can be administered by intravitreal (IVT) injection; targeting twice per year dosing Stereopure oligonucleotides open novel strategies in both dominant and recessive IRDs; potential for potent and durable effect with low immune response Successful targeting of MALAT1 is a surrogate for an ASO mechanism of action Widely expressed in many different cell types Only expressed in the nucleus Intravitreal injection Sources: Daiger S, et al. Clin Genet. 2013;84:132-141. Wong CH, et al. Biostatistics. 2018; DOI: 10.1093/biostatistics/kxx069. Athanasiou D, et al. Prog Retin Eye Res. 2018;62:1–23. Daiger S, et al. Cold Spring Harb Perspect Med. 2015;5:a017129. Verbakel S, et al. Prog Retin Eye Res. 2018:66:157-186.; Short, B.G.; Toxicology Pathology, Jan 2008. Ophthalmology

Durable Malat1 knockdown through 9 months with PN chemistry Compound or PBS (1 x 50 ug IVT) was delivered to C57BL6 mice. Relative percentage of Malat1 RNA in the posterior of the eye (retina, choroid, sclera) to PBS-treated mice is shown at 12, 20 and 36 weeks post-single injection. PBS = phosphate buffered saline; NTC= chemistry matched non-targeting control ~50% Malat1 knockdown at 36 weeks in the posterior of the eye PBS NTC PS/PO PS/PN % Malat1 expression Time (weeks) p≤0.01 Ophthalmology

Usher Syndrome Type 2A: a progressive vision loss disorder Autosomal recessive disease characterized by hearing loss at birth and progressive vision loss beginning in adolescence or adulthood Caused by mutations in USH2A gene (72 exons) that disrupt production of usherin protein in retina, leading to degeneration of the photoreceptors No approved disease-modifying therapies ~5,000 addressable patients in US Sources: Boughman et al., 1983. J Chron Dis. 36:595-603; Seyedahmadi et al., 2004. Exp Eye Res. 79:167-173; Liu et al., 2007. Proc Natl Acad Sci USA 104:4413-4418. Oligonucleotides that promote USH2A exon 13 skipping may restore production of functional usherin protein Ophthalmology

Potent USH2A exon 13 skipping with stereopure compound in vitro and ex vivo Left: Compounds were added to Y79 cells under free-uptake conditions. Exon skipping was evaluated by Taqman assays. USH2A transcripts were normalized to SRSF9. Data are mean±s.d., n=2. Reference Compound: van Diepen et al. 2018. Antisense oligonucleotides for the treatment of eye disease. W02018055134A1. Compound-1 is a stereopure antisense oligonucleotide. Right: Whole NHP and human eyes were enucleated (n=4 and n=2, respectively) and compounds (1–20 µM) were added to extracted retinas under free-uptake conditions. Exon skipping was evaluated by 48 hrs later by Taqman assays on RNA. USH2A transcript levels were normalized to SRSF9. Data presented are mean± s.e.m. Enhanced potency over a stereorandom reference compound (in vitro) Ophthalmology Target engagement in NHP and human retinas (ex vivo) PBS NTC Compound-1 20 20 10 5 1 [µM] PBS NTC Compound-1 20 20 10 [µM] NHP Human

Allele-selective reduction of SNP-containing allele for adRP associated with Rhodopsin P23H mutation Ferrari et al., Current Genomics. 2011;12:238-249.; Reporter assays on a Wave stereopure sequence as well as a sequence described in WO2016138353A1: ASO and luciferase reporter plasmids (wild-type and mutant rhodopsin) are transfected into Cos7 cells. 48-hours later, cells are harvested, and relative luminescence is measured. Stereorandom Stereopure Collaborations in place for evaluation in transgenic human Rho P23H pig model In vivo Ophthalmology Retinitis pigmentosa (RP): group of rare, genetic eye disorders resulting in progressive photoreceptor cell death and gradual functional loss; currently no cure ~10% of US autosomal dominant RP cases are caused by the P23H mutation in the rhodopsin gene (RHO) Mutant P23H rhodopsin protein is thought to misfold and co-aggregate with wild-type rhodopsin, resulting in a gain-of-function or dominant negative effect in rod photoreceptor cells

Expected upcoming milestones Huntington’s disease 4Q 2020: CTA submission for WVE-003 (SNP3) 1Q 2021: PRECISION-HD1 data, including 32 mg cohort, and initial data from OLE trial 1Q 2021: PRECISION-HD2 data, including 32 mg cohort, and initial data from OLE trial Amyotrophic lateral sclerosis and frontotemporal dementia 4Q 2020: CTA submission for WVE-004 (C9orf72) ALS: Amyotrophic lateral sclerosis; FTD: Frontotemporal dementia; CTA: clinical trial application; OLE: open-label extension Duchenne muscular dystrophy 1Q 2021: CTA submission for WVE-N531 (exon 53) Dosing in three new clinical trials expected in 2021 ADAR editing (Alpha-1 antitrypsin deficiency) 1H 2021: Humanized mouse model validation and in vivo data

Realizing a brighter future for people affected by genetic diseases For more information: Kate Rausch, Investor Relations krausch@wavelifesci.com 617.949.4827 Graham Morrell, Investor Relations gmorrell@wavelifesci.com 781.686.9600
